Potassium hydrogen phthalate (KHP) is used to standardize sodium hydroxide.If 35.39 mL of NaOH(aq) is required to titrate 0.8246 g KHP to the equivalence point,what is the concentration of the NaOH(aq) ? (The molar mass of KHP = 204.2 g/mol)
HC8H4O4-(aq) + OH-(aq) 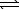 C8H4O42-(aq) + H2O(
C8H4O42-(aq) + H2O( 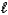 )
)
A) 0.02318 M
B) 0.05705 M
C) 0.0859 M
D) 0.1141 M
E) 0.1429 M
Correct Answer:
Verified
Q50: An impure sample of sodium carbonate,Na2CO3,is titrated
Q51: A solution containing 10.mmol of CO32- and
Q52: Which is the best colored indicator
Q53: What color change is exhibited by phenolphthalein
Q54: What is the solubility product expression for
Q56: The solubility of barium carbonate (BaCO3)in water
Q57: A 25.00-mL sample of propionic acid,HC3H5O2,of unknown
Q58: A solution containing 10.mmol of CO32-and 5.0
Q59: A 25.0 mL sample of 0.10
Q60: Consider the titration of 300.0 mL
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents