Calculate rG for the following reaction at 425 C,
2 HI(g) 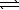 H2(g) + I2(g)
H2(g) + I2(g)
Given K = 0.018.(R = 8.314 J/K.mol)
A) 6.12 103 J
B) 1.05 104 J
C) 1.42 104 J
D) 2.33 104 J
E) 3.34 105 J
Correct Answer:
Verified
Q56: The change in entropy for any process
Q61: Does the formation of complex molecules such
Q66: At what temperature (in kelvin units)is the
Q67: The standard free energy change associated
Q68: The standard free energy change for
Q69: The solubility product equilibrium constant,Ksp,of silver
Q71: In any chemical process,energy must be conserved.This
Q72: What is the equilibrium constant for
Q75: The standard free energy of formation
Q76: Consider the following reaction:
2C(s)+ 2H2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents