Calculate 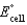 for the electrochemical cell below,
for the electrochemical cell below,
Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s)
Given the following reduction half-reactions.
Pb2+(aq) + 2 e- Pb(s) E = -0.126 V
PbCl2(s) + 2 e- Pb(s) + 2 Cl-(aq) E = -0.267 V
Fe3+(aq) + e- Fe2+(aq) E = +0.771 V
Fe2+(aq) + e- Fe(s) E = -0.44 V
A) -0.504 V
B) -0.062 V
C) +0.504 V
D) +1.038 V
E) +1.604 V
Correct Answer:
Verified
Q43: What is the copper(II)-ion concentration at 25°C
Q44: For the electrochemical cell Zn(s)| Zn2+ ||
Q45: The following electrochemical cell has a
Q46: What is the pH of the
Q47: A Faraday,F,is defined as
A) the charge on
Q49: Given the following two half-reactions,write the
Q50: The following has a potential of
Q51: Calculate the cell potential,at 25
Q52: The cell potential of the following
Q53: For the cell reaction ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents