For the cell reaction 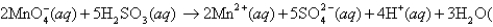 l )
l )
The standard cell potential is 1.34 V.To determine the cell potential at nonstandard conditions,what is the value that should be used for n in the Nernst equation?
A) 8
B) 10
C) 5
D) 2
E) 6
Correct Answer:
Verified
Q48: Calculate Q49: Given the following two half-reactions,write the Q50: The following has a potential of Q51: Calculate the cell potential,at 25 Q52: The cell potential of the following Q54: Calculate E°cell for the cell for Q55: What is the standard cell potential Q56: Calculate Ecell for the following electrochemical Q57: Which of the following statements is Q58: What is the value of the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents