Calculate the energy released (per mole of deuterium consumed) for the following fusion reaction, 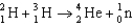 given the following molar masses of nucleons and nuclei.(c = 2.998 108 m/s)
given the following molar masses of nucleons and nuclei.(c = 2.998 108 m/s) 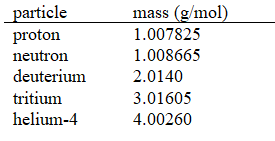
A) 5.63 106 J/mol
B) 1.69 1015 J/mol
C) 4.62 1013 J/mol
D) 8.44 1011 J/mol
E) 1.69 1012 J/mol
Correct Answer:
Verified
Q24: When Q25: When the radioactive nuclide Q26: By what (single step)process does polonium-218 Q27: The bismuth-209 nucleus has a binding Q28: Calculate the mass defect for an atom Q30: Radioactive isotopes with greater than 83 protons Q31: By what (single step)process does americium-241 Q32: What is the nuclear binding energy Q33: A certain radioactive isotope has a Q34: Which of the following nuclides has the![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents