Which of the following compounds has a dipole moment? Indicate the direction of the dipole moment. 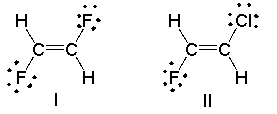
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q158: What is the approximate bond angle around
Q159: What is the hybridization state and molecular
Q160: What is the hybridization state and molecular
Q161: Which of the following compounds has a
Q162: Which of the following compounds have the
Q164: Which of the following statements best explains
Q165: BF3 has a no dipole moment. Draw
Q166: Which of the following compounds have a
Q167: Which intermolecular force is generally considered the
Q168: Which of the following compounds have the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents