Using Table 6.1, which of the following is the enthalpy change of the following reaction under standard conditions? 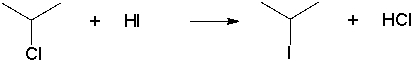
A) -21 kJ/mol
B) +21 kJ/mol
C) -171 kJ/mol
D) +171 kJ/mol
Correct Answer:
Verified
Q13: Predict the sign of ∆S for the
Q14: In a heterolytic bond cleavage, _ are
Q15: You are working in a research laboratory
Q16: Later in the course, we will compare
Q17: Why is the entropy change negative for
Q19: In a homolytic bond cleavage, _ are
Q20: Of following reactions, which one(s) would you
Q21: Does a reaction with a positive ∆S
Q22: What is the energy of activation for
Q23: Does a reaction with a ∆H of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents