You are working in a research laboratory and have developed a new reagent that cleaves C-H bonds homolytically. Unfortunately, this reagent can achieve only one of the transformations shown below. In light of bond dissociation energies, which transformation is most likely to be achieved?
A) 
B) 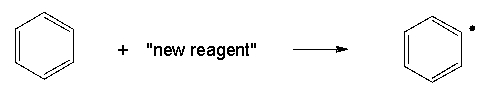
C) 
D) 
Correct Answer:
Verified
Q10: Using Table 6.1, estimate the enthalpy change
Q11: Later in the course, we will compare
Q12: Using Table 6.1, which of the following
Q13: Predict the sign of ∆S for the
Q14: In a heterolytic bond cleavage, _ are
Q16: Later in the course, we will compare
Q17: Why is the entropy change negative for
Q18: Using Table 6.1, which of the following
Q19: In a homolytic bond cleavage, _ are
Q20: Of following reactions, which one(s) would you
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents