Which point on the following diagram can be extrapolated to identify the length and strength of a chemical bond? 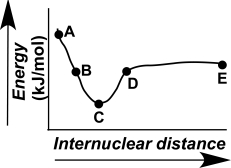
A) A
B) B
C) C
D) D
E) E
Correct Answer:
Verified
Q1: An atom of which element would have
Q2: Identify which carbon atom in the molecule
Q3: Which electron configuration is correct for the
Q4: Which of the following is an example
Q6: A C-O single bond is 143 pm
Q7: Identify which carbon atom in the molecule
Q8: Which line structure is correct for Molecule
Q9: Consider the interesting structure below,called a dibromocarbene.The
Q10: Which electron configuration is correct for a
Q11: Evaluate the Lewis structure below and determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents