Consider the interesting structure below,called a dibromocarbene.The carbon of the dibromocarbene has one lone electron pair and two separate covalent bonds to individual bromine atoms.What is the formal charge on the carbon atom of the dibromocarbene? 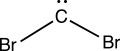
A) +2
B) +1
C) 0
D) -1
E) -2
Correct Answer:
Verified
Q4: Which of the following is an example
Q5: Which point on the following diagram can
Q6: A C-O single bond is 143 pm
Q7: Identify which carbon atom in the molecule
Q8: Which line structure is correct for Molecule
Q10: Which electron configuration is correct for a
Q11: Evaluate the Lewis structure below and determine
Q12: Which orbital does not house core electrons
Q13: Which point on the following diagram represents
Q14: When two Lewis structures are related as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents