A carbonyl,the C 
O unit,is a component of many important functional groups.Consider the Lewis structures below.Convert the Lewis structures to line structures,showing all lone pairs.Indicate bond dipoles using the arrow method.Rank the structures for increasing partial positive charge.Predict which carbonyl carbon should have greatest partial positive charge,assuming that the chlorine lone pairs do not engage in resonance.Explain your answer. 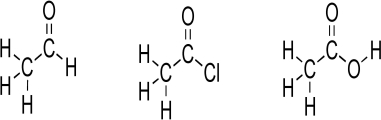
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q37: For which of the following
Q38: Which condensed formula contains an aldehyde functional
Q39: The evolution of a chemical bond can
Q40: In sum,how many total hydrogen atoms are
Q41: Identify the molecules below as monosaccharides,disaccharides,or carbohydrates.Explain
Q43: Are the Lewis structures for sulfuric acid
Q44: (a)Sulfuric acid,H2SO4,is an important strong oxo acid
Q45: Draw all possible resonance forms for anisole
Q46: The six unique hydrogen atoms in the
Q47: Global warming and ozone depletion both have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents