The six unique hydrogen atoms in the molecule below are labeled a-f.Suppose we individually replace each of these unique hydrogen atoms with a hydroxyl group (-OH)and draw a new molecule of formula C6H10O2.(In the cases of Ha,Hb,and Hc,substitute only one designated H with an OH.)Which of the new -OH groups would have localized lone pairs on oxygen? Which of the -OH groups would have a delocalized lone pair? Finally,for each molecule,identify the new functional group created. 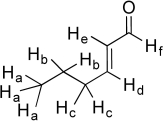
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q41: Identify the molecules below as monosaccharides,disaccharides,or carbohydrates.Explain
Q42: A carbonyl,the C Q43: Are the Lewis structures for sulfuric acid Q44: (a)Sulfuric acid,H2SO4,is an important strong oxo acid Q45: Draw all possible resonance forms for anisole Q47: Global warming and ozone depletion both have Q48: Consider a peptide bond formed from the Q49: To which carbon atoms in anisole would Q50: Peptide bonds are the building blocks of Q51: Identify the functional groups present in the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents