Propanol can be dissolved in diethyl ether and treated with sodium hydride,a base,to form a sodium alkoxide,as shown below. 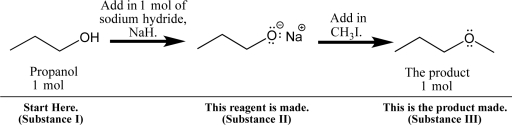
Although this and related chemical reactions will be studied in later chapters,you can apply your knowledge of solubility to the process.
Fill in your solubility predictions using the table below.Do you expect propanol (I),sodium propoxide (II),and the new ether product (III)to be soluble in the reaction solvent,diethyl ether? If soluble,predict the intermolecular interactions that will exist in solution.Also,anticipate the physical appearance of the reaction at each point: Will the solution be transparent,or will a precipitate form?

Correct Answer:
Verified
Q39: Which of the following intermolecular forces is
Q40: Identify the strongest intermolecular force.
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced
Q41: Rank N-N-dimethylaniline,phenethylamine,and phenethylamine hydrochloride in order of
Q42: Explain the chemical difference between a detergent
Q43: Which of the following benzene derivatives would
Q45: Your lab partner disobeyed lab rules and
Q46: (a) Group I cations are common ions
Q47: Rank 1,4-dimethylbenzene,phenol,and N,N-dimethylaniline in order of decreasing
Q48: Why do polar aprotic solvents solvate cations
Q49: Explain why polar protic solvents (like butanol)solvate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents