The molecule benzene,which features a conjugated ring system,has interesting properties.In fact,extended benzene-like molecules are key features of many drug classes,including DNA intercalators.
(a) Draw an energy-based electron configuration diagram for any one carbon atom in benzene.Clearly show which orbitals are hybridized and which orbital is used for the π bond.
(b) Next,consider the π MO diagram of benzene generated via the LCAO method.Add π electrons to the diagram,and label each orbital as π bonding MO or π* antibonding MO.How many total electrons reside in MOs of π symmetry for benzene? 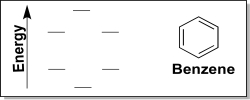
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q46: Draw line structures of a molecule with
Q47: Acetonitrile,C2H3N,is a polar aprotic solvent commonly used
Q48: Sketch an orbital picture of ethylene,H2C
Q49: Formaldehyde,CH2O,is a biological preservative and the simplest
Q50: Draw line structures of a molecule with
Q52: Use your knowledge of hybridization to fill
Q53: Compare the p orbital orientation and overlap
Q54: Consider the hybrid structure below and the
Q55: Tropyllium bromide is an ionic organic compound
Q56: Below are two reasonable acyclic structures for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents