Tropyllium bromide is an ionic organic compound that is soluble in water but not in diethyl ether.What is the hybridization of the positively charged carbon in tropyllium bromide? Do you expect the carbon framework to be planar? Justify your response using an orbital diagram. 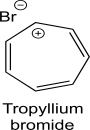
Correct Answer:
Verified
Q46: Draw line structures of a molecule with
Q47: Acetonitrile,C2H3N,is a polar aprotic solvent commonly used
Q48: Sketch an orbital picture of ethylene,H2C
Q49: Formaldehyde,CH2O,is a biological preservative and the simplest
Q50: Draw line structures of a molecule with
Q51: The molecule benzene,which features a conjugated ring
Q52: Use your knowledge of hybridization to fill
Q53: Compare the p orbital orientation and overlap
Q54: Consider the hybrid structure below and the
Q56: Below are two reasonable acyclic structures for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents