Using line structures,deduce individual resonance contributors from the resonance hybrid structure given here.Identify any lone pairs that are localized,rather than delocalized.Based on orbital hybridization theory,what orbitals accommodate these lone pairs? 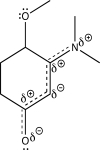
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q40: Which two atomic orbitals overlap to form
Q41: Bioymifi is a novel small molecule that
Q42: Amide bonds join amino acids together to
Q43: Tubulysin D is a peptide-based marine natural
Q44: Why is a σ bond formed from
Q46: Draw line structures of a molecule with
Q47: Acetonitrile,C2H3N,is a polar aprotic solvent commonly used
Q48: Sketch an orbital picture of ethylene,H2C
Q49: Formaldehyde,CH2O,is a biological preservative and the simplest
Q50: Draw line structures of a molecule with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents