What is the major driving force for the reaction below? 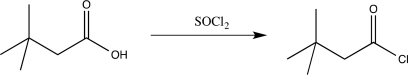
A) The product, an acid chloride, is more thermodynamically stable than a carboxylic acid.
B) Chloride is a better nucleophile than hydroxide.
C) The reaction is highly exothermic.
D) Hydroxide is a better leaving group than chloride.
E) The byproducts SO2 and HCl bubble out of solution irreversibly.
Correct Answer:
Verified
Q2: What is the role of PCl3 in
Q3: Which of the following correctly explains why
Q4: The reaction below is an example of
Q5: Which of the following compounds would undergo
Q6: What is the product of the following
Q8: Which of the following elementary steps is
Q9: Which of the following is an intermediate
Q10: How many proton transfer steps occur in
Q11: What base would be the best choice
Q12: What is the rate-determining step in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents