Which of the following correctly explains why two equivalents of amine are required for the aminolysis reaction shown below,while alcoholysis requires only one equivalent of alcohol? 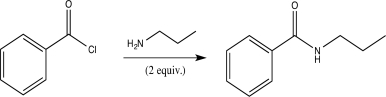
A) Amines are less nucleophilic than alcohols.
B) One equivalent of amine acts as a base and is irreversibly protonated.
C) Alcohols are stronger bases than amines.
D) Inductive effects from the Cl make the carbonyl C less electrophilic in aminolysis than in alcoholysis.
E) Two molecules of amine are involved in the rate-determining step.
Correct Answer:
Verified
Q1: Which of the following correctly explains how
Q2: What is the role of PCl3 in
Q4: The reaction below is an example of
Q5: Which of the following compounds would undergo
Q6: What is the product of the following
Q7: What is the major driving force for
Q8: Which of the following elementary steps is
Q9: Which of the following is an intermediate
Q10: How many proton transfer steps occur in
Q11: What base would be the best choice
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents