Which of the following correctly explains how sulfuric acid catalyzes the reaction shown below? 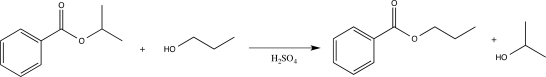
A) The acid increases the rate of nucleophile elimination.
B) The acid increases the nucleophilicity of the alcohol.
C) The acid donates a proton to the alcohol.
D) The acid converts the ester into a carboxylic acid.
E) The acid activates the carbonyl carbon and makes it more electrophilic.
Correct Answer:
Verified
Q2: What is the role of PCl3 in
Q3: Which of the following correctly explains why
Q4: The reaction below is an example of
Q5: Which of the following compounds would undergo
Q6: What is the product of the following
Q7: What is the major driving force for
Q8: Which of the following elementary steps is
Q9: Which of the following is an intermediate
Q10: How many proton transfer steps occur in
Q11: What base would be the best choice
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents