Sodium chlorate is used as an oxidizer in the manufacture of dyes,explosives and matches.Calculate the mass of solute needed to prepare 1.575 L of 0.00250 M NaClO3 ( 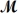
= 106.45 g/mol) .
A) 419 g
B) 169 g
C) 0.419 g
D) 0.169 g
E) 0.00394 g
Correct Answer:
Verified
Q6: Lithium hydroxide is used in alkaline batteries.
Q7: A 0.150 M sodium chloride solution is
Q9: Sodium hydroxide, also known as caustic soda,
Q41: How many grams of oxygen are
Q44: Potassium chlorate (used in fireworks, flares,
Q46: Phosphine, an extremely poisonous and highly
Q46: Magnesium reacts with iron(III)chloride to form
Q51: Lead(II) sulfide was once used in glazing
Q55: Aluminum oxide (used as an adsorbent
Q70: Methanol (CH4O) is converted to bromomethane
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents