Magnesium reacts with iron(III) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron.
3Mg(s) + 2FeCl3(s) 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 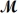
= 24.31 g/mol) and 175 g of iron(III) chloride ( 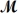
= 162.2 g/mol) is allowed to react.What mass of magnesium chloride = 95.21 g/mol) is formed?
A) 68.5 g MgCl2
B) 77.0 g MgCl2
C) 71.4 g MgCl2
D) 107 g MgCl2
E) 154 g MgCl2
Correct Answer:
Verified
Q7: A 0.150 M sodium chloride solution is
Q41: What is the percent yield for
Q43: Tetraphosphorus hexaoxide ( Q44: Aluminum reacts with oxygen to produce Q44: Potassium chlorate (used in fireworks, flares, Q46: Phosphine, an extremely poisonous and highly Q50: Sodium chlorate is used as an oxidizer Q51: Lead(II) sulfide was once used in glazing Q55: Aluminum metal reacts with chlorine gas to Q70: Methanol (CH4O) is converted to bromomethane![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents