Aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent,desiccant or catalyst for organic reactions.
4Al(s) + 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 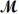
= 26.98 g/mol) and 117.65 g of oxygen ( 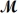
= 32.00 g/mol) is allowed to react.Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
A) Oxygen is the limiting reactant;19.81 g of aluminum remain.
B) Oxygen is the limiting reactant;35.16 g of aluminum remain.
C) Aluminum is the limiting reactant;16.70 g of oxygen remain.
D) Aluminum is the limiting reactant;35.16 g of oxygen remain.
E) Aluminum is the limiting reactant;44.24 g of oxygen remain.
Correct Answer:
Verified
Q22: Gadolinium oxide, a colorless powder which absorbs
Q38: Hydroxylamine nitrate contains 29.17 mass % N,
Q41: What is the percent yield for
Q43: Tetraphosphorus hexaoxide ( Q44: Potassium chlorate (used in fireworks, flares, Q46: Magnesium reacts with iron(III)chloride to form Q46: Phosphine, an extremely poisonous and highly Q51: Lead(II) sulfide was once used in glazing Q55: Aluminum metal reacts with chlorine gas to Q70: Methanol (CH4O) is converted to bromomethane![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents