What is the percent yield for the reaction
PCl3(g) + Cl2(g) PCl5(g)
If 119.3 g of PCl5 ( 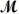
= 208.2 g/mol) are formed when 61.3 g of Cl2 ( 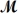
= 70.91 g/mol) react with excess PCl3?
A) 195%
B) 85.0%
C) 66.3%
D) 51.4%
E) 43.7%
Correct Answer:
Verified
Q22: Gadolinium oxide, a colorless powder which absorbs
Q38: Hydroxylamine nitrate contains 29.17 mass % N,
Q39: Alkanes are compounds of carbon and hydrogen
Q43: Tetraphosphorus hexaoxide ( Q44: Aluminum reacts with oxygen to produce Q46: Magnesium reacts with iron(III)chloride to form Q51: Lead(II) sulfide was once used in glazing Q53: How many grams of sodium fluoride Q54: In the combustion analysis of 0.1127 g Q55: Aluminum metal reacts with chlorine gas to![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents