Use Hess's Law to calculate the enthalpy change for the reaction
WO3(s) + 3H2(g) W(s) + 3H2O(g)
From the following data: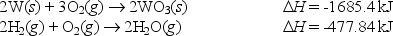
A) 125.9 kJ
B) 252.9 kJ
C) 364.9 kJ
D) 1207.6 kJ
E) None of these choices is correct.
Correct Answer:
Verified
Q26: Which one of the following is
Q27: Galena is the ore from which elemental
Q29: Calculate q when 28.6 g of
Q30: When Karl Kaveman adds chilled grog to
Q33: If, as a pioneer, you wished to
Q33: Use the following data to calculate
Q34: The Starship Enterprise is caught in a
Q35: Calcium hydroxide,which reacts with carbon dioxide to
Q36: A piece of copper metal is initially
Q55: Which one of the following statements about
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents