Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. 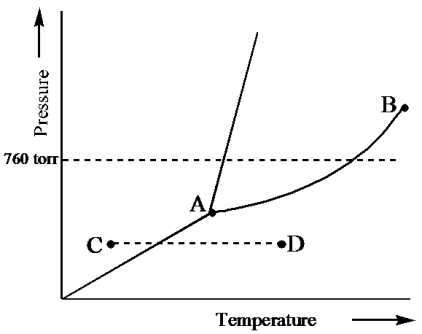
A) Bo(s) has a lower density than Bo(l) .
B) The triple point for Bo is at a higher temperature than the melting point for Bo.
C) Bo changes from a solid to a liquid as one follows the line from C to D.
D) Bo changes from a liquid to a gas as one follows the line from C to D.
E) Point B represents the critical temperature and pressure for Bo.
Correct Answer:
Verified
Q2: Examine the following phase diagram and identify
Q3: Diethyl ether,used as a solvent for extraction
Q4: Examine the following phase diagram and identify
Q5: Neon condenses due to
A)dipole-dipole forces.
B)London dispersion forces.
C)hydrogen
Q6: Examine the following phase diagram and determine
Q8: Octane has a vapor pressure of 40.torr
Q11: Liquid sodium can be used as
Q12: Liquid ammonia (boiling point = -33.4°C)can be
Q18: Pentane, C5H12, boils at 35°C. Which
Q24: In hydrogen iodide_ are the most important
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents