Benzaldehyde ( 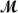
= 106.1 g/mol) ,also known as oil of almonds,is used in the manufacture of dyes and perfumes and in flavorings.What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? Kf = 1.99°C/m,freezing point of pure ethanol = -117.3°C.
A) -117.5°C
B) -118.7°C
C) -119.0°C
D) -120.6°C
E) < -121°C
Correct Answer:
Verified
Q10: If the density of a solution is
Q52: Barbiturates are synthetic drugs used as sedatives
Q58: Calculate the vapor pressure of a solution
Q60: Carbon tetrachloride,once widely used in fire extinguishers
Q76: Which of the following aqueous liquids will
Q87: A 0.100 m MgSO4 solution has a
Q93: A 50.0 % (w/w) solution of sulfuric
Q96: A 0.100 m K2SO4 solution has a
Q98: Octane and nonane are liquids which are
Q100: The density of pure water at 25°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents