Multiple Choice
Barbiturates are synthetic drugs used as sedatives and hypnotics.Barbital ( 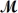
= 184.2 g/mol) is one of the simplest of these drugs.What is the boiling point of a solution prepared by dissolving 42.5 g of barbital in 825 g of acetic acid?
Kb = 3.07°C/m,boiling point of pure acetic acid = 117.9°C
A) 117.0°C
B) 117.7°C
C) 118.1°C
D) 118.8°C
E) >120°C
Correct Answer:
Verified
Related Questions
Q47: Calculate the freezing point of a solution
Q49: Hexachlorophene is used as a disinfectant in
Q51: Diethyl ether has a vapor pressure of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents