The correct formula for calculating mass percent of X in compound XY is: 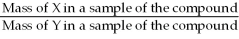
= Mass % X
Correct Answer:
Verified
Q21: The molecular formula is equal to the
Q23: C2H3O2 could be an empirical formula.
Q25: C2H6O4 could be an empirical formula.
Q27: One mole of lead(II)nitrate contains six moles
Q31: Which of the following statements about the
Q32: There are 6 grams of carbon in
Q32: The chemical formula CuBr2 indicates that this
Q33: An empirical formula gives the smallest whole
Q36: One half of a mole of atoms
Q38: What is the correct value for Avogadro's
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents