What is the theoretical yield in grams of CuS for the following reaction given that you start with 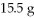
Of Na2S and 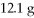
CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) 0.0758
B) 0.198
C) 18.93
D) 7.25
E) not enough information
Correct Answer:
Verified
Q101: Consider the reaction: 2 Al + 3Br2
Q104: Hydrochloric acid reacts with barium hydroxide according
Q105: How many grams of water are needed
Q107: How many grams of the excess reactant
Q109: If the theoretical yield of the reaction
Q110: How many moles of water are needed
Q111: If the theoretical yield of the reaction
Q112: What is the percent yield of CuS
Q113: Consider a reaction of chemicals depicted as
Q114: Consider the following reaction: 2 Mg +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents