Hydrochloric acid reacts with barium hydroxide according to the equation: 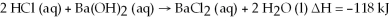
Calculate the heat (in kJ) associated with the complete reaction of 18.2 grams of HCl (aq) .
A) -58.9
B) +58.9
C) -29.5
D) -236
E) none of the above
Correct Answer:
Verified
Q84: In order to determine the limiting reactant
Q99: Which is the excess reactant in the
Q101: Starting with 156 g Q101: Consider the reaction: 2 Al + 3Br2 Q104: Consider the following equation: CO + 2 Q105: How many grams of water are needed Q107: How many grams of the excess reactant Q108: What is the theoretical yield in grams Q109: If the theoretical yield of the reaction Q113: Consider a reaction of chemicals depicted as![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents