Multiple Choice
Which is the excess reactant in the following reaction given that you start with 15.5 g of Na2S and 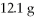
CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) Na2S
B) CuSO4
C) Na2SO4
D) CuS
E) not enough information
Correct Answer:
Verified
Related Questions
Q83: How many grams of water are theoretically
Q84: In order to determine the limiting reactant
Q88: How many grams of aluminum oxide are
Q91: How many moles of aluminum oxide are
Q96: Which is the limiting reactant in the
Q98: What is the excess reactant for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents