Starting with 156 g 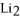
O and 33.3 g 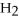
O,decide which reactant is present in limiting quantities.
Given: 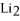
O + 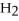
O → 2 LiOH
A) lithium oxide
B) water
C) lithium hydroxide
D) insufficient data
E) none of the above
Correct Answer:
Verified
Q84: In order to determine the limiting reactant
Q88: How many grams of aluminum oxide are
Q96: Which is the limiting reactant in the
Q98: What is the excess reactant for the
Q99: Which is the excess reactant in the
Q101: Consider the reaction: 2 Al + 3Br2
Q104: Consider the following equation: CO + 2
Q104: Hydrochloric acid reacts with barium hydroxide according
Q105: How many grams of water are needed
Q113: Consider a reaction of chemicals depicted as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents