Given that the freezing point depression constant for water is 1.86°C kg/mol,calculate the change in freezing point for a 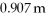
Sugar solution.
A) 0.488°C
B) 2.05°C
C) 0.592°C
D) 1.69°C
E) none of the above
Correct Answer:
Verified
Q102: An osmosis cell is constructed of a
Q103: Which of the following aqueous solutions would
Q108: What will happen if a healthy red
Q114: Osmotic pressure is:
A)the pressure required to stop
Q115: Which of the following aqueous solutions has
Q117: Which of the following statements about colligative
Q119: Calculate the molarity of a KCl solution
Q120: How many mL of 0.112 M Pb(N
Q120: Solution A has a concentration of 0.10
Q121: Calculate the molality of a solution prepared
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents