Consider the image below which shows a model of the compound known as hexane. The large spheres represent carbon and the smaller spheres represent hydrogen. 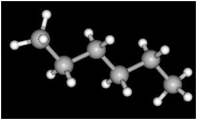
-The total number of single bonds in hexane is__________.
Correct Answer:
Verified
Q68: What is the solvent in a solution
Q69: Consider the following two Lewis dot structures
Q70: The following image shows two molecules of
Q71: Which of the following is a nonpolar
Q72: Choose the statement that best explains why
Q74: What is the molecular geometry of carbon
Q75: Negative ions are approximately the same size
Q76: Consider the following image. Q77: The dotted line in the following images Q78: A binary ionic compound generally consists of![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents