Consider the following model of ethanol, CH3CH2OH. 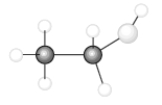 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-In the balanced equation for the combustion of one mole of ethanol, the coefficient of water is _____.
Correct Answer:
Verified
Q39: Which of the following compounds would you
Q40: A coal gasification process involving a catalyst,
Q41: The structure below represents an aromatic hydrocarbon
Q42: Consider the following model of ethanol, CH3CH2OH.
Q43: The following two hydrocarbon models represent structural
Q45: Which of the bond types shown below
Q46: Identify the alkane shown below.
A) C5H12
B) C10H20
C)
Q47: Consider the following model of ethanol, CH3CH2OH.
Q48: Consider the following model of ethanol, CH3CH2OH.
Q49: Which of the bond types shown below
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents