Consider the following model of ethanol, CH3CH2OH. 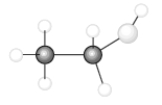 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-Using the data in the following table, 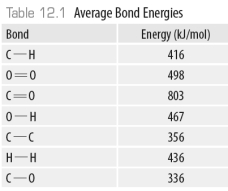 The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
Correct Answer:
Verified
Q42: Consider the following model of ethanol, CH3CH2OH.
Q43: The following two hydrocarbon models represent structural
Q44: Consider the following model of ethanol, CH3CH2OH.
Q45: Which of the bond types shown below
Q46: Identify the alkane shown below.
A) C5H12
B) C10H20
C)
Q48: Consider the following model of ethanol, CH3CH2OH.
Q49: Which of the bond types shown below
Q50: Which hydrocarbon would be isolated at the
Q51: The following compound could represent the principle
Q52: Identify the alkene shown below.
A) C5H12
B) C10H20
C)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents