In the figure below,which state of matter has the highest entropy? 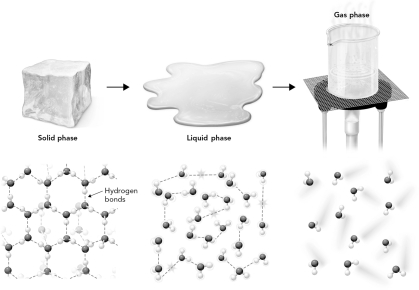
A) solid phase
B) liquid phase
C) gas phase
D) all are equal entropy.
Correct Answer:
Verified
Q12: The difference between an oxidation reaction and
Q13: The example of water freezing into ice
Q14: Which of the following correctly describes the
Q15: Which of the following is an example
Q16: The change in entropy of a system
Q18: The oxidation of glucose releases 15.7 kJ/g.Is
Q19: A hot pack on your arm is
Q20: For a given reaction with a
Q21: Hydrogen bonds in liquid water are formed
Q22: The standard free energy change is defined
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents