For a given reaction with a 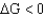 ,the reaction is
,the reaction is
A) favorable in the reverse direction.
B) favorable in the forward direction.
C) unfavorable in both directions.
D) favorable in both directions.
Correct Answer:
Verified
Q15: Which of the following is an example
Q16: The change in entropy of a system
Q17: In the figure below,which state of matter
Q18: The oxidation of glucose releases 15.7 kJ/g.Is
Q19: A hot pack on your arm is
Q21: Hydrogen bonds in liquid water are formed
Q22: The standard free energy change is defined
Q23: Given the energy charge equation below,if a
Q24: If the equilibrium constant (Keq)is greater than
Q25: In a hydrogen bond between a water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents