Calculate the pH of a solution that contains 3.9 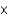 10-4M H+.
10-4M H+.
A) 4.59
B) 10.59
C) 3.41
D) 9.41
Correct Answer:
Verified
Q41: Which of the following acids is the
Q42: Weak acids have a high pKa because
Q43: How do plants,fungi,and bacteria avoid the damaging
Q44: What is the concentration of H+ in
Q45: Which of the following is the Kw
Q47: If an unknown solution has low pKa
Q48: An organism in equilibrium with its environment
Q49: Red blood cells are placed into a
Q50: Limonene is a nonpolar molecule.The water molecules
Q51: What is the expected osmotic pressure around
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents