Which of the following acids is the strongest given their Ka values?
A) HF (3.5 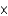 10-4)
10-4)
B) HClO2 (1.1 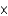 10-2)
10-2)
C) HCN (4.9 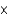 10-10)
10-10)
D) HNO2 (4.6 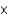 10-4)
10-4)
Correct Answer:
Verified
Q36: If Q37: If the equilibrium constant (Keq)is greater than Q38: The transfer of a phosphate from ATP Q39: If a reaction has a Q40: Under what conditions could a biological reaction Q42: Weak acids have a high pKa because Q43: How do plants,fungi,and bacteria avoid the damaging Q44: What is the concentration of H+ in Q45: Which of the following is the Kw Q46: Calculate the pH of a solution that![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents