Why is the 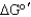 of the condensation of oxaloacetate and acetyl-CoA highly favorable?
of the condensation of oxaloacetate and acetyl-CoA highly favorable?
A) The addition of inorganic phosphate provides energy, resulting in the highly favorable nature of this reaction.
B) Allosteric regulations of the reaction cause a release of energy, making the reaction thermodynamically favorable.
C) The iron-sulfur cluster in the enzyme reduces the activation energy of the reaction, resulting in the favorable 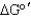
D) The hydrolysis of the thioester bond in citryl-CoA results in the highly exergonic reaction.
Correct Answer:
Verified
Q45: How is the pyruvate dehydrogenase reaction regulated?
A)
Q46: What enzyme in the citrate cycle is
Q47: How would a high NADH to NAD+
Q48: An in vitro study shows that
Q49: Which reaction in the citrate cycle produces
Q51: The reaction catalyzed by _ is likely
Q52: Which reaction in the citrate cycle produces
Q53: The reaction catalyzed by _ is the
Q54: A high concentration of which molecule would
Q55: The reaction catalyzed by _is the most
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents