Chlorofluorocarbons are important air pollutants implicated in global warming and ozone depletion. How fast does the chlorofluorocarbon CF2Cl2 diffuse relative to N2, the main component of the atmosphere?
In other words, calculate the ratio of diffusion rates: 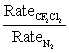
Correct Answer:
Verified
Q78: Which of the following changes will NOT
Q80: The volume of a single molecule of
Q81: Briefly state the conditions corresponding to STP
Q83: Aluminum metal shavings (10.0 g) are
Q84: Calculate the density in g/L of gaseous
Q85: In the fermentation process, yeast converts
Q87: At moderate pressures (~ 200 atm), the
Q87: Use the van der Waals equation for
Q95: Nitrogen will behave most like an ideal
Q97: If the molecular mass of a gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents