Consider the Figure Which Shows G° for a Chemical Process Plotted Against Absolute Temperature
Consider the figure which shows G° for a chemical process plotted against absolute temperature. 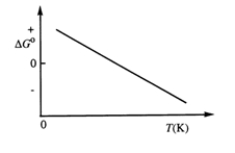 Which one of the following is an incorrect conclusion, based on the information in the diagram?
Which one of the following is an incorrect conclusion, based on the information in the diagram?
A) . H° > 0
B) . S° > 0
C) The reaction is spontaneous at high temperatures.
D) . S° increases with temperature while H° remains constant.
E) There exists a certain temperature at which H° = T S°.
Correct Answer:
Verified
Q54: The second law of thermodynamics tells us
Q61: Given: C2H2(g)
Q62: A chemical reaction has
Q63: What is the free energy change,
Q64: A reaction is proceeding toward equilibrium.
Q66: For each of the following pairs, predict
Q67: a. Explain what is meant by a
Q68: A reaction has
Q69: In the expression, S = k ln
Q70: The reaction of methane with water to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents