For each of the following pairs, predict which (A or B) will have the greater entropy, and in one sentence indicate your reasoning. 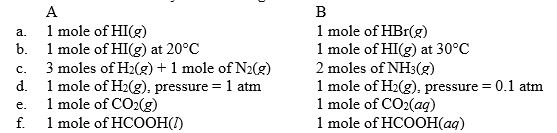
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q61: Given: C2H2(g)
Q62: A chemical reaction has
Q63: What is the free energy change,
Q64: A reaction is proceeding toward equilibrium.
Q65: Consider the figure which shows
Q67: a. Explain what is meant by a
Q68: A reaction has
Q69: In the expression, S = k ln
Q70: The reaction of methane with water to
Q71: Consider the figure which shows
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents