Consider the reaction of iodine with manganese dioxide 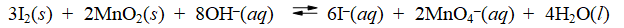
The equilibrium constant for the overall reaction is 8.30 × 10-7. Calculate E°cell for the reaction at 25°C.
A) -0.36 V
B) -0.18 V
C) -0.12 V
D) -0.060 V
E) none of the above
Correct Answer:
Verified
Q55: What is the value of the equilibrium
Q56: Consider the non-aqueous cell reaction 2Na(l)
Q57: The value of E°cell for the
Q58: Consider the reaction in the lead-acid
Q59: A concentration cell consists of two Zn/Zn2+
Q63: Which of the following elements could be
Q72: Which of the following elements can be
Q77: In the electrolysis of aqueous sodium sulfate
Q84: A solution is prepared by dissolving 32.0
Q90: What product forms at the cathode during
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents