Refer to the figure below. 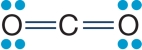 The figure shows the molecular structure of carbon dioxide.Carbon dioxide is nonpolar, whereas water is polar.Which of the true statements below explains these differences?
The figure shows the molecular structure of carbon dioxide.Carbon dioxide is nonpolar, whereas water is polar.Which of the true statements below explains these differences?
A) Carbon dioxide does not contain any polar covalent bonds, whereas water does.
B) Carbon dioxide contains only double bonds, whereas water contains only single bonds.
C) Carbon dioxide is a linear molecule, whereas water has a bent shape.
D) Carbon dioxide contains carbon atoms, whereas water does not.
E) Carbon and oxygen do not differ greatly in electronegativity, whereas hydrogen and oxygen do.
Correct Answer:
Verified
Q39: Drawings of hydrogen, deuterium, and tritium would
Q40: Differences in the electronegativity of atoms that
Q41: A covalent bond is formed by the
Q42: Carbon-14 is a radioactive isotope of carbon.When
Q43: Which statement about biochemical reactions is false?
A)
Q45: Refer to the oxidation-reduction reaction below.
Q46: A chemist measures the masses of two
Q47: All of the following are nonpolar except
A)
Q48: Hydrogen bonds are attractions
A) between oppositely charged
Q49: Refer to the figure below. 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents