Refer to the figure below. 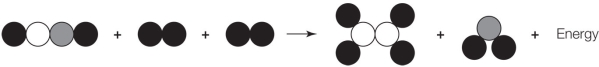 Which statement about the figure is true?
Which statement about the figure is true?
A) It shows a chemical change because the products differ from the reactants.
B) It shows a chemical change because the three molecules were transformed into two molecules.
C) It shows a chemical change because energy was released as a result of the change.
D) It does not accurately show a chemical change because the numbers of atoms on the two sides of the arrow differ.
E) It does not accurately show a chemical change because energy is shown on the wrong side of the arrow.
Correct Answer:
Verified
Q44: Refer to the figure below. 
Q45: Refer to the oxidation-reduction reaction below.
Q46: A chemist measures the masses of two
Q47: All of the following are nonpolar except
A)
Q48: Hydrogen bonds are attractions
A) between oppositely charged
Q50: A van der Waals interaction is an
Q51: Some reactions, such as the decomposition of
Q52: Hydrogen bonds
A) form between two hydrogen atoms.
B)
Q53: When magnesium (Mg) bonds with another element,
Q54: Which compound is held together by ionic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents