What type of chemical bond connects the two carbon atoms in the molecule shown below? 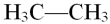
A) Nonpolar covalent bond
B) Asymmetric bond
C) Polar covalent bond
D) Hydrogen bond
E) Double covalent bond
Correct Answer:
Verified
Q142: Which is not an example of a
Q143: Refer to the figure below. 
Q144: A single carbon atom can bond a
Q145: What type of chemical bond connects the
Q146: The five statements below describe properties of
Q148: Covalent bonds
A) include polar but not nonpolar
Q149: A neon atom has a total of
Q150: Which structure represents an isotope of hydrogen?
A)
Q151: Refer to the figure below. 
Q152: In the periodic table, when elements are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents