Refer to the figure below. 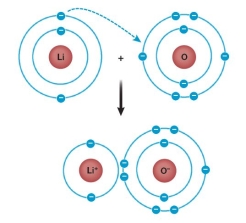 Does this figure accurately show how the ionic compound lithium oxide forms?
Does this figure accurately show how the ionic compound lithium oxide forms?
A) Yes, because it shows two atoms forming ions that attract one another.
B) Yes, because it shows how lithium and oxygen share electrons to form a bond.
C) No, because lithium should gain electrons from oxygen, not the other way around, as shown.
D) No, because oxygen has an incomplete outer shell and needs an electron from a second lithium atom.
E) No, because the figure does not show how lithium and oxygen share electrons.
Correct Answer:
Verified
Q163: Which is an instance of van der
Q164: Refer to the figure below. 
Q165: Refer to the figure below. 
Q166: Which statement is false?
A) Covalent bonds can
Q167: Which experimental procedure could be used to
Q169: Refer to the figure below. 
Q170: A cation has the opposite charge from
Q171: The electronegativity of an atom is a
Q172: Which statement provides evidence that living organisms
Q173: Vegetable oil is composed of long-chain hydrocarbon
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents