Refer to the figure below. 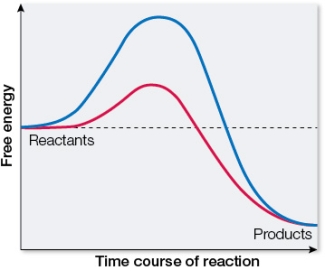 This graph shows the change in free energy for the same reaction with and without a catalyst.Which tracing represents the catalyzed reaction and why?
This graph shows the change in free energy for the same reaction with and without a catalyst.Which tracing represents the catalyzed reaction and why?
A) The blue tracing represents the catalyzed reaction because it has a larger G than the uncatalyzed reaction.
B) The blue tracing represents the catalyzed reaction because it has a larger activation energy than the uncatalyzed reaction.
C) The red tracing represents the catalyzed reaction because it has a smaller G than the uncatalyzed reaction.
D) The red tracing represents the catalyzed reaction because it has a smaller activation energy than the uncatalyzed reaction.
E) The red tracing represents the catalyzed reaction because it leads to the formation of more products than the uncatalyzed reaction.
Correct Answer:
Verified
Q161: A theoretical enzyme, anyase, catalyzes the same
Q162: The term referring to a reactant in
Q163: Which series best summarizes the relative energies
Q164: Which statement best describes the mechanism
Q165: An enzyme
A) decreases the
Q167: Enzymes are biological catalysts and function by
A)
Q168: Transition-state intermediates have higher energies than the
Q169: The conversion of A to B, which
Q170: Refer to the figure below. 
Q171: Hexokinase is the enzyme that catalyzes the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents